During the 1960s, two independent groups identified a compound active in the initiation of bud dormancy in sycamore and cotton boll abscission, naming it dormin and abscisin II, respectively.
Following its purification from cotton fruits, the chemical structure of this compound was determined in 1965 and it was renamed abscisic acid (ABA).
Shortly after this, it was discovered that ABA levels increase considerably when plants wilt and that ABA causes stomatal closure. These two discoveries highlighted the importance of ABA in mediating responses of vegetative tissues to environmental stresses such as drought, high salinity,and low temperature.
ABA is also required for the accumulation of seed nutrient reserves, the acquisition of desiccation tolerance, and the arrest of embryonic development during seed maturation. Despite its name, ABA is not a major regulator of absicission, which is primarily controlled by ethylene.
STRUCTURE AND OCCURRENCE
Like all hormones, ABA responses depend not only on the sensitivity of the tissue to ABA, but also on local ABA concentration. This is regulated by the biosynthesis, degradation, inactivation, transport, and subcellular compartmentation of the hormone.
A. STRUCTURE
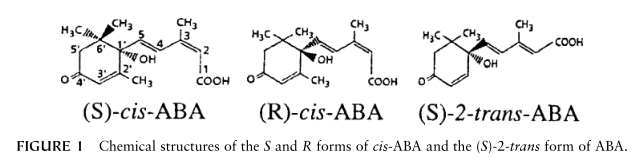
The 15 carbon atoms of the sesquiterpene ABA configure an aliphatic ring with one double bond, three methyl groups, and an unsaturated chain containing the carboxyl group (Fig. 1).
The cis and trans isomers differ in the orientation of the carboxyl group, and the asymmetric carbon at the 1’ position of the ring distinguishes between the S(+) and R(-) enantiomers.
The exact ABA chemical structure is essential for its physiological activity, and the loss of a carboxyl group, a tertiary hydroxyl group, a 2-cis 4-trans-pentadienoic side chain, a 4'-ketone, or a double bond in the cyclohexane ring greatly reduces activity.
B. OCURRENCE
ABA is widespread in vascular plants, occurring in mosses, ferns, liverworts (where a similar compound, lunaric acid, plays a similar role), and all algal classes, including photosynthetic prokaryotes such as cyanobacteria.
Some pathogenic fungi make ABA, but the biosynthetic pathway appears to be quite different from that of higher plants. ABA is also reported to occur in the mammalian brain, although its role there is not known.
SYNTHESIS
The endogenous ABA concentration can rise and fall dramatically in response to environmental or developmental cues. It appears that ABA is synthesized in almost all cells containing chloroplasts or amyloplasts (i.e., plastids), but the regulatory controls appear to differ between tissues.
Not only do absolute ABA concentrations increase dramatically during embryogenesis, but the ABA content of leaves and roots increases 10- to 50-fold when water potentials fall below - 1.0 MPa (approximately - 10.0 bar).
The concentration of ABA in the xylem sap of well-watered plants is 1.0–15.0 nM and can increase to 3.0 μM after water stress. The main rise in ABA caused by water loss occurs some 2–3 hour after the onset of wilting The ability of cycloheximide to block this process indicates a requirement for de novo protein synthesis and thus implicates an up-regu- lation of ABA biosynthesis in stressed tissues.
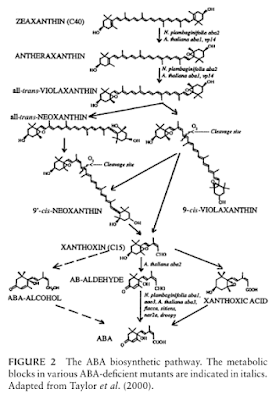
The plant ABA biosynthetic pathway represents a minor branch of the carotenoid pathway and begins in plastids. In contrast with isoprene biosynthesis in animal cells, the main precursor of ABA, isopentenyl diphosphate (IPP), is generated by the methyl erythritol phosphate pathway and not from mevalo- nic acid.
Eight IPP residues are combined to form geranylgeranyl diphosphate, the precursor for the biosynthesis of the C40 compound b-carotene. Both rings of b-carotene are hydroxylated to form the xanthophyll zeaxanthin, which can be regarded as the first intermediate in ABA synthesis (Fig. 2).
The role of xanthophylls as intermediates in ABA biosynthesis is supported by the reduced ABA content of maize vp (viviparous) mutants that are blocked in early steps of carotenoid synthesis.
Zeaxanthin is then oxidized to antheraxanthine and to all-trans-violaxanthin by zeaxanthin epoxidase (ZEP), which is absent in the Nicotiana plumbaginifolia aba2 mutant.
ZEP contains a putative N-terminal transit sequence for targeting to chloroplasts. ABA2/ZEP expression is detected in stems, leaves, roots, and seeds and it is strongly induced by drought stress in roots but not leaves.
The oxidative cleavage of the 9-cis-epoxycarote- noid precursor generates the 15C skeleton of ABA.
The maize vp14 mutant is deficient in the chloroplas- tic 9-cis-epoxycarotenoid dioxygenase (NCED) responsible for the cleavage of the 9-cis-isomers, but not the all-trans-isomers, to xanthoxin.
Therefore, the precise order of isomerization-type reactions remains uncertain. The gene is expressed constitutively in embryos and roots and in contrast with ABA2/ZEP transcripts, NCED transcripts accumu- late to high levels in water-stressed leaves. Thus, ABA accumulation in wilted leaves is primarily regulated at the level of plastidic xanthoxin production, which appears to be rate-limiting in ABA biosynthesis.
Transgenic experiments indicated that ZEP is primarily involved in the regulation of ABA synthesis during seed development. The final steps are not yet completely defined. Xanthoxin is converted to ABA in the cytosol via either AB-aldehyde or xanthoxic acid. Genetic evidence suggests that xanthoxin is first oxidized to AB-aldehyde, although the involvement of xanthoxic acid as a precursor has not been completely eliminated.
Arabidopsis thaliana aba3 and tomato flacca and sitiens mutants are defective in the last oxidation step and are thus unable to oxidize AB-aldehyde to ABA. This last step involves an enzyme that requires a molybdenum cofactor. Arabidopsis aba3 and N. plumbaginifolia aba1 mutants cannot produce the functional molybdate cofactor required by AB-aldehyde oxidase (AO).
This last enzyme of ABA synthesis is not highly substrate-specific and interest- ingly, AO can also catalyze synthesis of another plant growth regulator, the auxin indole-3-acetic acid. In Arabidopsis, a multigene family comprising at least four members encodes AO, only one of which appears to act specifically in ABA synthesis.
Alternative Pathways
Although ABA2 is a single copygene,the ABA content of the N. plumbaginifolia aba2 null mutant is 23–48% that of the wildtype (WT). Moreover, ABA is present in tomato flacca and sitiens mutants that lack an effective AO. These results suggest either that there is more than one biosynthetic pathway or that there is some redundancy in part of the primary biosynthetic pathway.
The2-trans-ABA-alcohol accumulated during water stress in flacca and sitiens mutants could be formed via a P450 mono-oxygenase able to add a second oxygen atom to the C1 position and can be further slowly converted to ABA.
This reaction may also occur to a small extent in WT plants. Unlike plants,fungi are able to synthesize ABA directly from the 15C compound farnesyl pyrophosphate.
DEGRADATION
After wilted leaves regain turgor, ABA can be inactivated by oxidation to phaseic acid and dihy- drophaseic acid or by conjugation to glucose to form a glucose ester. In the first case, catabolic inactivation proceeds via hydroxylation at the 8 0 position to form an unstable intermediate that subsequently forms phaseic acid.
The ABA-8 0 -hydroxylase considered as the pivotal enzyme in ABA degradation is a mem- brane-associated cytochrome P450 mono-oxygenase. It is expressed at high levels in plant tissues recovering from hyperosmotic stresses.
Although phaseic acid is still able to trigger stomatal closure in some species, its activity is much weaker than that of ABA. In contrast, dihydrophaseic acid, which is the reduced form of phaseic acid, has no detectable activity.
Conjugation of ABA to glucose not only renders ABA inactive but also changes its distribution in the cell. Whereas free ABA is mainly cytosolic, ABA-b- D - glycosyl ester accumulates in vacuoles and could be a storage form of the hormone. Until now, neither the enzymes involved in ABA catabolism nor the genes that encode them have been isolated.
TRANSPORT
ABA is secreted by cells into the apoplast (i.e., intercellular space) and is easily transported in both xylem and phloem sap to most plant parts, especially stems, leaves, roots, and ripening fruits. Since roots are the primary sites of perception of water deficit, ABA synthesized in roots can be transported to shoot tissues via the transpiration stream, where it triggers stomatal closure to reduce water loss from leaves.
Within leaves,ABA is redistributed as a function of pH. In a well-watered plant, the xylem sap is more acidic (approximately pH 6.3) and ABA occurs in its protonated form (ABAH). During drought stress, the sap becomes slightly alkaline (approximately pH 7.2), favoring the deprotonation of ABAH to ABA. As a result, less ABA is taken up by mesophyll cells and more is diverted to guard cells. Therefore, even though absolute ABA levels may not change, the pH-dependent redistribution of root-derived ABA to guard cells can induce stomatal closure.
A similar redistribution may exist within cells. When photosynthesis is active, the pH of the chloroplast stroma increases as protons are pumped into the thylakoid lumen. A prevalence of deproto- nated ABA limits its ability to cross the chloroplast membrane, causing the accumulation of ABA in the stroma.
During drought stress, photosynthetic rates decrease. The resulting drop in stromal pH increases levels of ABAH, which can traverse membranes and be released for transport to guard cells.
ROLES OF ABA
ABA is unquestionably involved in a plant’s response to stress and in the initiation and maintenance of seed dormancy. However, it also influences many other aspects of plant physiology, often by interacting synergistically/antagonistically with hormones such as ethylene, gibberellins, cytokinins, auxin, jasmonic acid, and brassinosteroids or by modulating meta- bolic sensing pathways such as those monitoring cellular sugar status.
A. ABA Triggers Stomatal Closure
During Water Stress Stomata are pores, found on the aerial surfaces of plants, that allow CO 2 uptake for photosynthesis and at the same time the loss of water, which drives the transpiration stream. Stomatal pore diameter is regulated by turgor changes of the two surrounding guard cells. Unlike most other cells in higher-plant tissues, the absence of plasmodesmata in mature guard cells renders them independent of surrounding cells and enables them to respond autonomously to stimuli such as CO 2 , water status, temperature, red/blue light, and plant pathogens.
Applied ABA inhibits the opening and promotes the closure of stomata. The increased transpiration rates observed in ABA-biosynthetic mutants and the accumulation of ABA in stressed leaves with reduced transpiration are consistent with the view that endogenous ABA normally plays an important role in the reduction of water loss by transpiration.
Expression of an anti- ABA antibody in transgenic tobacco plants retained ABA in the endoplasmic reticulum and caused leaves to wilt by impairing stomatal closure. The molecular mechanism by which ABA induces stomatal closure has been studied using genetic, biochemical, single-cell, and electrophysiological approaches. Opening and closing of stomata is thought to provoke turgor and volume changes in guardcells.
During water stress,the increase in cellular ABA or in apoplastic ABA at guard cell surfaces mediates guard cell closure by triggering a net efflux of K þ and Cl 2 from the vacuole to the cytoplasm and from the cytoplasm to the apoplast. Additionally, sucrose and malate are metabolized to osmotically inactive starch, all of which function to reduce the osmolarity in the guard cells.
During stomatal opening, guard cells swell following the accumulation of K þ , anions, and sucrose. The resulting out-bowing of the guard cell pair increases pore aperture and allows reestablishment of transpiration.
B. ABA Promotes Seed Maturation and Dormancy
One of the clearest effects of ABA is to prepare the seed for desiccation and to impose embryo dormancy to prevent premature germination. Seeds of ABA-deficient mutants or transgenic plants depleted of endogenous ABA by expression of an ABA-specific antibody fail to mature fully and acquire dormancy.
Seed development can be divided into two phases of equal duration. The first includes growth and development of the embryo and the endosperm. The second phase begins with the arrest of cell division and the accumulation of storage reserves and is followed by preparation for desiccation, which occurs in the last stages of seed maturation.
Seeds prepare for desiccation by accumulating nutritive reserves and proteins that allow the cell to survive the ensuing loss of up to 90% of its water content. As a consequence of dehydration, seeds become dormant.
The ABA content of seeds increases during the first half of seed development and decreases during the second phase involving seed maturation. ABA strongly induces genes that encode abun- dantly expressed seed storage proteins (e.g., zein, conglycinin, and lectin proteins) as well as proteins involved in desiccation tolerance.
The highly conserved water-soluble and basic late-embryogenesis abundant (LEA) proteins are rich in glycine/lysine and low in hydrophobic residues and are thought to stabilize other proteins when the cell is dehydrated. They are related to members of the DHN (dehydrin) and RAB (Responsive to ABA) protein families.
C. ABA Inhibits Germination and Seedling Growth
Seed germination can be defined as the resumption of growth of the embryo following dormancy. As possibly the most critical developmental transition in the plant life cycle, germination is contingent on suitable environmental conditions.
However, dormant seeds will not germinate even under normally permissive conditions of temperature or water, light, and oxygen availability.
Seed dormancy introduces a delay in germination that provides additional time for geographical dispersal and also maximizes seedling survival by preventing germination under adverse conditions. ABA appears to be the most important mediator of seed dormancy.
The ability of exogenous ABA to prevent seed germination in many species has been used to isolate several abi (ABA-insensitive) Arabi- dopsis mutants (Table 1). Exogenous ABA can also inhibit the precocious germination of immature embryos in culture. ABA-deficient Arabidopsis (aba) mutants are nondormant at maturity, and embryos of maize vp mutants germinate directly on the cob while still attached to the mother plant.
This precocious germination, named vivipary, suggests that ABA normally constrains developing embryos in an early developmental stage. In contrast with maize, ABA deficiency in Arabidopsis does not cause vivipary because the rigid seed coat prevents embryo growth while the seed is in the seed pod. Nevertheless, vivipary occurs when Arabidopsis abi3 mutant embryos are dissected out of the seed coat before complete desiccation.
Germination is regulated by an antagonism between ABA, which promotes dormancy, and gibberellic acid (GA), which counteracts the effects of ABA by promoting growth and the mobilization of storage reserves. An elegant demonstration of this antagonism is the recovery of mutants defective in ABA synthesis in a screen for revertants of GA- deficient mutants.
D. ABA Controls Root and Shoot Growth
ABA shows different effects on root and shoot growth depending on plant water status. Under water stress, ABA depresses both shoot and root growth, but the overall effect is a dramatic increase in the root:shoot ratio, which facilitates water conservation.
TABLE 1. Mutations Affecting ABA Biosynthesis and Signal Transduction Pathway in Plants
Plant species Mutations
E. ABA Mediates Wound Responses
After mechanical wounding, a specific set of defense- related proteins, such as protease inhibitors I and II, cathepsin D inhibitor, and threonine deaminase, accumulate both at the site of injury and systemically throughout the plant. ABA, together with jasmonic acid, appears to play a role in the induction of these genes.
THE ABA SIGNAL TRANSDUCTION PATHWAY
Clues as to how the ABA signal is transduced to mediate its physiological and developmental pro- cesses are now beginning to emerge. It should be emphasized that although many individual com- ponents have been identified mainly by molecular genetic approaches, the complete network has not yet been elucidated.
A. Receptor(s)
ABA is thought to initiate its effects by binding to a receptor(s) that triggers the signal transduction cascade.
Currently, the identity of the receptor(s) is unknown. Cells may possess at least two sites of ABA perception, one of which is located at the plasma membrane and is triggered by extracellular ABA. Biophysical studies indicate that ABA effects in stomatal guard cells also involve intracellular recep- tors accessible to the protonated form, ABAH, which readily permeates membranes.
B. Downstream Signaling Events
Recently, considerable insights have been gained into the identities of molecular components of the com- plex signaling network that mediates the actions of ABA. In particular, ion channels and fairly ubiquitous small second messengers have been implicated in ABA action.
1. Ion Channels Regulated by ABA Control Stomatal Aperture
Electrophysiological studies, either by whole cell impalement or by patch clamping of the plasma membrane of guard cell protoplasts or isolated vacuoles, have identified a number of membrane ion channels.
The sequence of events in ABA-induced stomatal closure is thought to be the following:
(1) ABA induces release of Ca 2+ into the cytosol from an internal store, e.g., the vacuole.
(2) The resultant increase in cytosolic Ca 2+ inhibits plasma membrane H + pumps and inward K + (K) in + channels, but activates two types of plasma membrane anion-efflux channels. One of these shows voltage-dependent slow activation (S-type), whereas the other shows rapid transient activation (R-type). The two types may reflect different states of a single channel.
(3) The conjugate actions of these channels lead to a transient or sustained depolarization and the alkalinization of the guard cell cytoplasm, which
(4) deactivates K + in + channels and also contributes to the opening of voltage-gated K + out channels. The ensuing long-term efflux of both anions and K + from guard cells contributes to loss of turgor and to stomatal closure.
2. Ca 21 Channels
Considerable evidence indicates that ABA produces repetitive, transient increases or oscillations in intracellular Ca 2+ levels. These encode information required for stomatal closure. Ca 2+ induced [Ca 2+ ] cyt oscillations include a repetitive Ca 2+ influx across the plasma membrane coupled to Ca 2+ release from an intracellular compartment. Stomatal closure is abol- ished in guard cells when a nonoscillating Ca 2+ plateau is imposed experimentally.
Many other stimuli responsible for stomatal closure (cold shock, oxidative stress, and increases in CO 2 ) also cause [Ca 2+ ] cyt elevations. Anion channel regulation and stomatal movement phenotypes of Arabidopsis abi1-1 or abi2-1 mutants are suppressed by experimentally elevating [Ca 2+ ] cyt .
The mechanisms by which ABA activates guard cell plasma membrane Ca 2þ channels remain unknown. In Arabidopsis guard cells, ABA causes a rapid increase of reactive oxygen species (ROS) that activate hyperpolarization-activated Ca 2+ -permeable channels. ROS-induced stomatal closure and Ca 2+ activation are abolished in the ABA-insensitive mutant gca2.
The origin of the Ca 2+ required to elevate [Ca 2+ ] cyt in response to ABA is unclear, but is probably mediated by inositol 1,4,5-trisphosphate (InsP 3 ) and/or cyclic ADP-ribose (cADPR). RAB18 expression in Arabidopsis suspension culture cells requires rapid ABA-induced Ca 2+ influx and S-type anion channel activation. ABA-induced membrane depolarization in radish seedlings and tobacco epidermal and mesophyll cells indicates that these mechanisms are of general importance for ABA signaling in different cell types. A Ca 2+ -independent pathway also appears to exist.
3. H 1 Channels
Inhibition of the plasma membrane H þ -ATPase mediated by both cytosolic alkalinization and the increase in cytosolic Ca 2+ may also contribute to ABSCISIC ACID 6 membrane depolarization. The origin of ABA- induced cytosolic alkalinization is unknown.
4. Cyclic Nucleotides (cAMP, CGMP, CADPR)
Cyclic ADP-ribose (cADPR) plays a central role in ABA responses. Microinjection of hypocotyl cells of the tomato aurea mutant with both potential intermediates in the ABA signaling cascade and fusions of the Arabidopsis RD29A and KIN2 promoters to a reporter gene suggested that ABA triggers a transient accumulation of cADPR, which induces a release of Ca 2+ from internal stores such as vacuoles and the endoplasmic reticulum. Microinjec- tion of mutant abi1-1 protein inhibited ABA-, cADPR-, and Ca 2+ -induced gene expression, and these effects were reversed by an excess of WT ABI1 protein.
Other cyclic nucleotides may also act in a Ca 2þ - dependent stomatal opening pathway. For example, cAMP or the membrane-permeable cyclic GMP analog 8-Br-cGMP stimulates stomatal opening. cGMP-induced stomatal opening is inhibited by chelation of external Ca 2þ or by inhibitors of intracellular Ca 2þ release.
5. Lipid-Derived Second Messengers
Various lines of evidence suggest that ABA stimulates phosphoinositide metabolism. ABA-treated guardcell protoplasts showed a slight increase in InsP 3 . The release of caged InsP 3 into the cytosol of guard cells caused [Ca 2+ ] cyt increases, inhibition of K þ in channels, and stomatal closure.
The Arabidopsis fry1 (fiery1) mutant, which is defective in an inositol polyphos- phate 1-phosphatase, accumulates more InsP 3 than WT plants after ABA treatment and is hypersensitive to ABA in germination and gene expression assays. Similarly, overexpression of a different InsP 3 phos- phatase blocked the inhibition of germination and seedling growth in Arabidopsis.
Overexpression of a stress- and ABA-inducible phosphatidylinositol- specific phospholipase C (PI-PLC) in Arabidopsis suggests that although increased InsP 3 levels are necessary for maximal ABA-induced gene expression in vegetative tissues, the AtPLC1 isoform is normally latent and probably participates in secondary ABA responses.
A reduction in InsP 3 levels in transgenic lines expressing antisense AtPLC1 correlated with their insensitivity to ABA in germination and seedling growth assays. ABA also stimulates production of myo-inositol- hexakisphosphate (InsP 6 ) in guard cells to a greater extent than InsP 3 . InsP 6 inhibits K + in channels in a Ca 2+ -dependent manner with greater efficiency than InsP 3 . Whether InsP 6 causes [Ca 2+ ] cyt elevations and whether both messengers function in the same or separate signaling branches is unknown.
Phosphatidic acid (PtdOH) generated from phos- pholipase D (PLD) increases transiently following ABA treatment of Vicia faba guard cells. PtdOH promotes stomatal closure and inactivates K þ in chan- nel currents but does not elicit a [Ca 2þ ] cyt increase, suggesting that PLD acts either in a parallel Ca 2þ - independent pathway or downstream of Ca 2þ release.
Both U-73122 (a PI-PLC inhibitor) and 1-butanol (a PLD inhibitor) only partially inhibit ABA-dependent stomatal closure, and simultaneous application of both inhibitors does not have additive effects. Thus, PLC and PLD appear to act in the same pathway that requires the cooperation of an additional pathway(s) to attain the complete effects of ABA.
This may be mediated by cADPR, since simultaneous application of 1-butanol with the cADPR antagonist nicotina- mide increases the extent to which ABA-induced stomatal closure is reduced. Sphingosine-1-phosphate (S1P) is another lipid- derived Ca 2þ -mobilizing agent capable of inducing stomatal closure. An increase in S1P levels occurs in leaves following drought stress, but disruption of S1P production causes only partial inhibition of ABA- induced stomatal closure.
6. Protein Kinases and Protein Phosphatases
Phosphorylation/dephosphorylation events are cen- tral mediators in ABA signaling. In guard cells, the Ca 2+ signal is possibly relayed by specific protein kinases and phosphatases.
Protein kinase inhibitors abolish the activation of S-type anion channels and thus block ABA-induced stomatal closure. Reciprocally, the protein phosphatase inhibitor okadaic acid (OKA) maintains guard cell S-type channels in the active state. In-gel phosphorylation assays demon- strated that ABA rapidly activates a Ca 2+ -indepen- dent 48 kDa Ser/Thr protein kinase in V. faba guard cells. ABA fails to activate anion channels or induce stomatal closure in guard cells that express a dominant loss-of-function allele of this kinase.
Several stress- and ABA-inducible protein kinases have been identified. In epidermal peels of Pisum sativum, the ABA-induced accumulation of a DHN transcript was reduced by K-252a (an inhibitor of Ser/Thr protein kinases) and also by OKA or cyclosporin A (an inhibitor of Ser/Thr protein phosphatases type 2B). In barley aleurone proto- plasts, the stimulation of a MAP kinase activity appeared to be correlated with the induction of RAB16 transcript. OKA inhibited the induction ABSCISIC ACID 7 of HVA1 and RAB16 transcripts by ABA, and phenylarsine oxide (an inhibitor of Tyr protein phosphatases) blocked RAB16 induction.
The analysis of abi1 and abi2 has shed new light on the involvement of phosphorylation events in ABA signaling. These two dominant ABA-insensitive mutants, originally isolated in a screen for mutants able to germinate and grow in nonpermissive ABA concentrations, have phenotypes reminiscent of ABA deficiency viz. reduced seed dormancy, improper regulation of stomatal aperture, and decreased expression of various ABA-inducible genes. ABI1 and ABI2 encode Ser/Thr protein phosphatases type 2C (PP2C).
The dominant mutant alleles abi1-1 and abi2-1 have point mutations that substitute a conserved Gly with Asp, probably disrupting the conformation of a site required for Mg 2þ -binding or phosphatase activity. Several downstream responses to ABA are impaired in abi1-1 and abi2-1, including K þ out and K þ in channel regulation, anion channel activation, and increases in [Ca 2+ ] cyt . Because the mutations are dominant, it remains unclear whether the ABI1 and ABI2 are positive or negative regulators of ABA signaling or, indeed, whether they affect ABA signaling at all in WT plants.
However, because intragenic revertants of abi1-1 and abi2-1 have reduced or no phosphatase activity in vitro and a double mutant of both revertants is hypersensitive to ABA, ABI1 and ABI2 are probably negative regulators of ABA signaling.
Accordingly, overexpression of WTABI1 in maize mesophyll protoplasts blocks ABA regulation of gene expression. The precise roles of kinases and phosphatases in ABA signaling and the identities of their protein substrates have not been clearly established.
7. Farnesylation
Although researchers have focused mainly on posi- tively acting components of the ABA signaling pathway, inactivation of negative regulators of ABA signaling should result in an enhanced response to ABA. An Arabidopsis mutant era1 (enhanced response to ABA) was isolated based on its inability to germinate in the presence of low concentrations of ABA (0.3 mM) that do not inhibit germination of WT seeds.
The era1 mutation markedly increases seed dormancy and ABA hypersensitive activation of S-type anion currents in this mutant increases stomatal closure, reducing water loss during drought. The ERA1 gene encodes the b-subunit of a hetero- dimeric farnesyltransferase. Farnesyltransferases cat- alyze the attachment of a 15-carbon farnesyl lipid to C-terminal target sequences, which localizes specific soluble signaling proteins to membranes. In addition to enhancing ABA signaling, loss of ERA1 function affects several other signaling pathways and develop- mental programs, including meristem development.
Thus, although ERA1 targets are not restricted to ABA action, a factor that normally suppresses ABA responses requires farnesylation. The exact relation- ship between ERA1 and the ABI loci remains unknown.
8. RNA Binding and ABA
Arabidopsis abh1 mutants are hypersensitive to ABA- mediated inhibition of germination as well as induction of stomatal closure and increases in [Ca 2þ ] cyt . ABH1 is expressed in stomata and encodes a nuclear transcript cap-binding protein that appar- ently functions in a heterodimeric complex. ABH1, by analogy to yeast and mammalian RNA cap- binding proteins, is proposed to regulate the strength of ABA signaling by transcript modification of early signaling components.
9. Heterotrimeric G-Protein Action
Heterotrimeric G-proteins are central to many signaling processes. Arabidopsis contains only a single gene (GPA1) encoding a prototypic Ga subunit. ABA-mediated inhibition of stomatal open- ing, but not ABA-controlled promotion of stomatal closure, is impaired in gpa1 null mutants. GPA1 is required for negative regulation of K þ in channels and the pH-independent activation of anion channels.
10. Role of the Actin Cytoskeleton in Stomatal Movements
A reorganization of the actin cytoskeleton of guard cells has been observed after ABA treatment. Cyto- chalasin D (an actin filament-depolymerizing agent) activates K þ in channels, while phalloidin (an actin filament stabilizer) inhibits K þ channel currents. ABA treatments reorganize actin cytoskeleton architecture from a radial arrangement to a randomly oriented and fragmented pattern.
A small Arabidopsis GTP- binding protein, AtRac1, is a negative regulator in ABA-induced actin reorganization. The inactivation of AtRac1 by ABA is impaired in abi1-1.
C. Regulation of Gene Expression by ABA
ABA regulates the expression of numerous genes during embryogenesis and seed maturation as well as under stress conditions such as heat shock, low temperature, drought, and high salinity.
1. ABA-Inducible Genes
The use of ABA-deficient and ABA-insensitive mutants has demonstrated that ABA contributes to the regulation of numerous genes involved in seed maturationand/ortheresponseofvegetativetissuesto hyperosmotic stress.
Characterization of the promo- ters of ABA-responsive genes has enabled identifi- cation of the cis- and trans-acting elements that act at the termini of branches in the ABA signaling cascade. Considerable evidence indicates the existence of ABA-independent dehydration and cold-induced sig- naling pathways.
2. Cis-Acting Elements
Gene activation is mediated by the binding of transcription factors to ABA-responsive elements (ABREs) located in the promoters of ABA-induced genes. To date, more than 20 functional ABREs have been found in ABA-inducible genes that are abun- dantly expressed in desiccating seeds and/or are responsive to drought stress and ABA in vegetative tissues.
The first type of ABRE defined was a sequence of 8–10 bp that shares a conserved ACGT core motif, named the G-box. The sequence flanking the ACGT core is important for in vivo and in vitro function. Some ACGT elements confer developmental and tissue-specific expression on a minimal promoter. In a natural promoter context, an ABRE functions with a coupling element (CE).
ABA-responsive complexes comprising an ABRE and a CE can confer ABA- inducible transcription upon a minimal promoter. The sph element, first identified in the promoter of the C1 gene involved in anthocyanin synthesis in maize endosperm, is a second category of cis-acting element distinct from the G-box.
3. Trans-Acting Factors
Yeast one-hybrid assays to identify ABRE-binding proteins (AREBs) have enabled cloning of several homologous transcription factors of the basic leucine zipper (bZIP) family. ABA-regulated transcription factors of the homeodomain leucine zipper, basic helix-loop-helix leucine zipper, and MYB classes have also been identified. AREBS are capable of activating reporter genes fused to ABREs and their induction by ABA at the transcript level frequently precedes the inductionofotherABA-responsivegenes.
Arabidopsis ABI5, the only bZIP AREB recovered in a genetic screen, is also subject to post transcriptional modification by ABA. Maize VP1 and Arabidopsis ABI3 appear to be orthologous seed-specific transcriptional activators, the loss of which affects several aspects of seed maturation, including the expression of storage proteins and LEA genes. VP1/ABI3-like proteins appear to activate transcription by distinct mechanisms depending on the target cis elements. VP1 interacts directly with the sph element of the C1 promoter and acts on ABREs via association with a distinct trans-acting factor(s).
D. Novel Genetic Screens
Quantitative and mechanistic characterization of new signaling mutants is necessary for a complete molecular understanding of the ABA signaling cascade. Several mutants have been isolated in screens for deregulated ABA control of ABREs fused to reporter genes.
Eight gca (growth control by ABA) mutants are characterized by reduced sensitivity to the inhibition of seedling growth by exogenous ABA and aberrant stomatal regulation. An elegant screen that uses small differences in leaf temperature to distinguish transpiration rates in mutants and WT plants is likely to identify new elements which mediate ABA action in guard cells. Screens for enhancer or suppressor mutations offer one approach to identify genes which interact genetically with known participants in ABA signaling.
BIOTECHNOLOGICAL FEATURES
Fresh water scarcity is currently one of the principal threats to global food security. Plants account for approximately 65% of global fresh water use. Losses in agricultural yields resulting from the desiccation of crops and horticultural plants during periods of drought have severe social and economic repercussions. Unfortunately, because of the high cost of synthesis and its instability in UV light, there are no practical uses of ABA. However, synthetic ABA analogs such as the acetyleneacetal-type compounds LAB 173 711 and LAB 144143 reduce crop water use and increase cold-hardiness.
Engineering the ABA signal transduction network in guard cells to control CO 2 intake and water loss could contribute substantially to more sustainable water use under adverse environmental conditions. The manipulation of seed maturation and dormancy in certain species by modification of ABA-regulated developmental pro- grams may also be of considerable agricultural significance.
CONCLUSIONS
Recent advances have filled in many gaps concerning the biochemistry and subcellular localization of ABA synthesis as well as demonstrating the unquestionable involvement of ion channels, cytosolic pH, protein (de)phosphorylation, and cADPR- and phosphoinosi- tide-mediated increases in [Ca 2þ ] cyt in transducing the ABA signal.
Substantial progress has been made in characterization of the terminal signaling elements involved in ABA-mediated transcriptional regulation. Nonetheless, the mechanism(s) of ABA perception and early signaling events that result in cADPR synthesis or InsP 3 release remain to be resolved.
Considering the multitude of physiological responses modulated by ABA, it will be interesting to assess the extent of overlap in the signaling events involved in well-characterized ABA effects such as the regulation of stomatal closure, the inhibition of seed germina- tion, and the induction of stress-responsive gene expression.
The striking degree of phenotypic pleiotropy observed in many mutants recovered in screens for altered sensitivity to ABA indicates extensive overlap between ABA action and other signaling pathways. Better insight into the regulation of ABA concentrations and the cellular capacity for response to ABA will provide a more complete picture of its importance to growth and development throughout the plant life cycle.
Following its purification from cotton fruits, the chemical structure of this compound was determined in 1965 and it was renamed abscisic acid (ABA).
Shortly after this, it was discovered that ABA levels increase considerably when plants wilt and that ABA causes stomatal closure. These two discoveries highlighted the importance of ABA in mediating responses of vegetative tissues to environmental stresses such as drought, high salinity,and low temperature.
ABA is also required for the accumulation of seed nutrient reserves, the acquisition of desiccation tolerance, and the arrest of embryonic development during seed maturation. Despite its name, ABA is not a major regulator of absicission, which is primarily controlled by ethylene.
STRUCTURE AND OCCURRENCE
Like all hormones, ABA responses depend not only on the sensitivity of the tissue to ABA, but also on local ABA concentration. This is regulated by the biosynthesis, degradation, inactivation, transport, and subcellular compartmentation of the hormone.
A. STRUCTURE
The 15 carbon atoms of the sesquiterpene ABA configure an aliphatic ring with one double bond, three methyl groups, and an unsaturated chain containing the carboxyl group (Fig. 1).
The cis and trans isomers differ in the orientation of the carboxyl group, and the asymmetric carbon at the 1’ position of the ring distinguishes between the S(+) and R(-) enantiomers.
The different forms of ABA occur in different proportions in plants and can have different activities. The S-cis form is the most abundant naturally occurring form and is the active form in fast responses such as stomatal closure. Both enantiomers are active in long-term responses such as changes in gene expression and protein synthesis. In contrast to the cis-trans isomers, the S and R forms cannot be interconverted in planta.
The exact ABA chemical structure is essential for its physiological activity, and the loss of a carboxyl group, a tertiary hydroxyl group, a 2-cis 4-trans-pentadienoic side chain, a 4'-ketone, or a double bond in the cyclohexane ring greatly reduces activity.
B. OCURRENCE
ABA is widespread in vascular plants, occurring in mosses, ferns, liverworts (where a similar compound, lunaric acid, plays a similar role), and all algal classes, including photosynthetic prokaryotes such as cyanobacteria.
Some pathogenic fungi make ABA, but the biosynthetic pathway appears to be quite different from that of higher plants. ABA is also reported to occur in the mammalian brain, although its role there is not known.
SYNTHESIS
The endogenous ABA concentration can rise and fall dramatically in response to environmental or developmental cues. It appears that ABA is synthesized in almost all cells containing chloroplasts or amyloplasts (i.e., plastids), but the regulatory controls appear to differ between tissues.
Not only do absolute ABA concentrations increase dramatically during embryogenesis, but the ABA content of leaves and roots increases 10- to 50-fold when water potentials fall below - 1.0 MPa (approximately - 10.0 bar).
The concentration of ABA in the xylem sap of well-watered plants is 1.0–15.0 nM and can increase to 3.0 μM after water stress. The main rise in ABA caused by water loss occurs some 2–3 hour after the onset of wilting The ability of cycloheximide to block this process indicates a requirement for de novo protein synthesis and thus implicates an up-regu- lation of ABA biosynthesis in stressed tissues.
The plant ABA biosynthetic pathway represents a minor branch of the carotenoid pathway and begins in plastids. In contrast with isoprene biosynthesis in animal cells, the main precursor of ABA, isopentenyl diphosphate (IPP), is generated by the methyl erythritol phosphate pathway and not from mevalo- nic acid.
Eight IPP residues are combined to form geranylgeranyl diphosphate, the precursor for the biosynthesis of the C40 compound b-carotene. Both rings of b-carotene are hydroxylated to form the xanthophyll zeaxanthin, which can be regarded as the first intermediate in ABA synthesis (Fig. 2).
The role of xanthophylls as intermediates in ABA biosynthesis is supported by the reduced ABA content of maize vp (viviparous) mutants that are blocked in early steps of carotenoid synthesis.
Zeaxanthin is then oxidized to antheraxanthine and to all-trans-violaxanthin by zeaxanthin epoxidase (ZEP), which is absent in the Nicotiana plumbaginifolia aba2 mutant.
ZEP contains a putative N-terminal transit sequence for targeting to chloroplasts. ABA2/ZEP expression is detected in stems, leaves, roots, and seeds and it is strongly induced by drought stress in roots but not leaves.
The oxidative cleavage of the 9-cis-epoxycarote- noid precursor generates the 15C skeleton of ABA.
The maize vp14 mutant is deficient in the chloroplas- tic 9-cis-epoxycarotenoid dioxygenase (NCED) responsible for the cleavage of the 9-cis-isomers, but not the all-trans-isomers, to xanthoxin.
Therefore, the precise order of isomerization-type reactions remains uncertain. The gene is expressed constitutively in embryos and roots and in contrast with ABA2/ZEP transcripts, NCED transcripts accumu- late to high levels in water-stressed leaves. Thus, ABA accumulation in wilted leaves is primarily regulated at the level of plastidic xanthoxin production, which appears to be rate-limiting in ABA biosynthesis.
Transgenic experiments indicated that ZEP is primarily involved in the regulation of ABA synthesis during seed development. The final steps are not yet completely defined. Xanthoxin is converted to ABA in the cytosol via either AB-aldehyde or xanthoxic acid. Genetic evidence suggests that xanthoxin is first oxidized to AB-aldehyde, although the involvement of xanthoxic acid as a precursor has not been completely eliminated.
Arabidopsis thaliana aba3 and tomato flacca and sitiens mutants are defective in the last oxidation step and are thus unable to oxidize AB-aldehyde to ABA. This last step involves an enzyme that requires a molybdenum cofactor. Arabidopsis aba3 and N. plumbaginifolia aba1 mutants cannot produce the functional molybdate cofactor required by AB-aldehyde oxidase (AO).
This last enzyme of ABA synthesis is not highly substrate-specific and interest- ingly, AO can also catalyze synthesis of another plant growth regulator, the auxin indole-3-acetic acid. In Arabidopsis, a multigene family comprising at least four members encodes AO, only one of which appears to act specifically in ABA synthesis.
Alternative Pathways
Although ABA2 is a single copygene,the ABA content of the N. plumbaginifolia aba2 null mutant is 23–48% that of the wildtype (WT). Moreover, ABA is present in tomato flacca and sitiens mutants that lack an effective AO. These results suggest either that there is more than one biosynthetic pathway or that there is some redundancy in part of the primary biosynthetic pathway.
The2-trans-ABA-alcohol accumulated during water stress in flacca and sitiens mutants could be formed via a P450 mono-oxygenase able to add a second oxygen atom to the C1 position and can be further slowly converted to ABA.
This reaction may also occur to a small extent in WT plants. Unlike plants,fungi are able to synthesize ABA directly from the 15C compound farnesyl pyrophosphate.
DEGRADATION
After wilted leaves regain turgor, ABA can be inactivated by oxidation to phaseic acid and dihy- drophaseic acid or by conjugation to glucose to form a glucose ester. In the first case, catabolic inactivation proceeds via hydroxylation at the 8 0 position to form an unstable intermediate that subsequently forms phaseic acid.
The ABA-8 0 -hydroxylase considered as the pivotal enzyme in ABA degradation is a mem- brane-associated cytochrome P450 mono-oxygenase. It is expressed at high levels in plant tissues recovering from hyperosmotic stresses.
Although phaseic acid is still able to trigger stomatal closure in some species, its activity is much weaker than that of ABA. In contrast, dihydrophaseic acid, which is the reduced form of phaseic acid, has no detectable activity.
Conjugation of ABA to glucose not only renders ABA inactive but also changes its distribution in the cell. Whereas free ABA is mainly cytosolic, ABA-b- D - glycosyl ester accumulates in vacuoles and could be a storage form of the hormone. Until now, neither the enzymes involved in ABA catabolism nor the genes that encode them have been isolated.
TRANSPORT
ABA is secreted by cells into the apoplast (i.e., intercellular space) and is easily transported in both xylem and phloem sap to most plant parts, especially stems, leaves, roots, and ripening fruits. Since roots are the primary sites of perception of water deficit, ABA synthesized in roots can be transported to shoot tissues via the transpiration stream, where it triggers stomatal closure to reduce water loss from leaves.
Within leaves,ABA is redistributed as a function of pH. In a well-watered plant, the xylem sap is more acidic (approximately pH 6.3) and ABA occurs in its protonated form (ABAH). During drought stress, the sap becomes slightly alkaline (approximately pH 7.2), favoring the deprotonation of ABAH to ABA. As a result, less ABA is taken up by mesophyll cells and more is diverted to guard cells. Therefore, even though absolute ABA levels may not change, the pH-dependent redistribution of root-derived ABA to guard cells can induce stomatal closure.
A similar redistribution may exist within cells. When photosynthesis is active, the pH of the chloroplast stroma increases as protons are pumped into the thylakoid lumen. A prevalence of deproto- nated ABA limits its ability to cross the chloroplast membrane, causing the accumulation of ABA in the stroma.
During drought stress, photosynthetic rates decrease. The resulting drop in stromal pH increases levels of ABAH, which can traverse membranes and be released for transport to guard cells.
ROLES OF ABA
ABA is unquestionably involved in a plant’s response to stress and in the initiation and maintenance of seed dormancy. However, it also influences many other aspects of plant physiology, often by interacting synergistically/antagonistically with hormones such as ethylene, gibberellins, cytokinins, auxin, jasmonic acid, and brassinosteroids or by modulating meta- bolic sensing pathways such as those monitoring cellular sugar status.
A. ABA Triggers Stomatal Closure
During Water Stress Stomata are pores, found on the aerial surfaces of plants, that allow CO 2 uptake for photosynthesis and at the same time the loss of water, which drives the transpiration stream. Stomatal pore diameter is regulated by turgor changes of the two surrounding guard cells. Unlike most other cells in higher-plant tissues, the absence of plasmodesmata in mature guard cells renders them independent of surrounding cells and enables them to respond autonomously to stimuli such as CO 2 , water status, temperature, red/blue light, and plant pathogens.
Applied ABA inhibits the opening and promotes the closure of stomata. The increased transpiration rates observed in ABA-biosynthetic mutants and the accumulation of ABA in stressed leaves with reduced transpiration are consistent with the view that endogenous ABA normally plays an important role in the reduction of water loss by transpiration.
Expression of an anti- ABA antibody in transgenic tobacco plants retained ABA in the endoplasmic reticulum and caused leaves to wilt by impairing stomatal closure. The molecular mechanism by which ABA induces stomatal closure has been studied using genetic, biochemical, single-cell, and electrophysiological approaches. Opening and closing of stomata is thought to provoke turgor and volume changes in guardcells.
During water stress,the increase in cellular ABA or in apoplastic ABA at guard cell surfaces mediates guard cell closure by triggering a net efflux of K þ and Cl 2 from the vacuole to the cytoplasm and from the cytoplasm to the apoplast. Additionally, sucrose and malate are metabolized to osmotically inactive starch, all of which function to reduce the osmolarity in the guard cells.
During stomatal opening, guard cells swell following the accumulation of K þ , anions, and sucrose. The resulting out-bowing of the guard cell pair increases pore aperture and allows reestablishment of transpiration.
B. ABA Promotes Seed Maturation and Dormancy
One of the clearest effects of ABA is to prepare the seed for desiccation and to impose embryo dormancy to prevent premature germination. Seeds of ABA-deficient mutants or transgenic plants depleted of endogenous ABA by expression of an ABA-specific antibody fail to mature fully and acquire dormancy.
Seed development can be divided into two phases of equal duration. The first includes growth and development of the embryo and the endosperm. The second phase begins with the arrest of cell division and the accumulation of storage reserves and is followed by preparation for desiccation, which occurs in the last stages of seed maturation.
Seeds prepare for desiccation by accumulating nutritive reserves and proteins that allow the cell to survive the ensuing loss of up to 90% of its water content. As a consequence of dehydration, seeds become dormant.
The ABA content of seeds increases during the first half of seed development and decreases during the second phase involving seed maturation. ABA strongly induces genes that encode abun- dantly expressed seed storage proteins (e.g., zein, conglycinin, and lectin proteins) as well as proteins involved in desiccation tolerance.
The highly conserved water-soluble and basic late-embryogenesis abundant (LEA) proteins are rich in glycine/lysine and low in hydrophobic residues and are thought to stabilize other proteins when the cell is dehydrated. They are related to members of the DHN (dehydrin) and RAB (Responsive to ABA) protein families.
C. ABA Inhibits Germination and Seedling Growth
Seed germination can be defined as the resumption of growth of the embryo following dormancy. As possibly the most critical developmental transition in the plant life cycle, germination is contingent on suitable environmental conditions.
However, dormant seeds will not germinate even under normally permissive conditions of temperature or water, light, and oxygen availability.
Seed dormancy introduces a delay in germination that provides additional time for geographical dispersal and also maximizes seedling survival by preventing germination under adverse conditions. ABA appears to be the most important mediator of seed dormancy.
The ability of exogenous ABA to prevent seed germination in many species has been used to isolate several abi (ABA-insensitive) Arabi- dopsis mutants (Table 1). Exogenous ABA can also inhibit the precocious germination of immature embryos in culture. ABA-deficient Arabidopsis (aba) mutants are nondormant at maturity, and embryos of maize vp mutants germinate directly on the cob while still attached to the mother plant.
This precocious germination, named vivipary, suggests that ABA normally constrains developing embryos in an early developmental stage. In contrast with maize, ABA deficiency in Arabidopsis does not cause vivipary because the rigid seed coat prevents embryo growth while the seed is in the seed pod. Nevertheless, vivipary occurs when Arabidopsis abi3 mutant embryos are dissected out of the seed coat before complete desiccation.
Germination is regulated by an antagonism between ABA, which promotes dormancy, and gibberellic acid (GA), which counteracts the effects of ABA by promoting growth and the mobilization of storage reserves. An elegant demonstration of this antagonism is the recovery of mutants defective in ABA synthesis in a screen for revertants of GA- deficient mutants.
D. ABA Controls Root and Shoot Growth
ABA shows different effects on root and shoot growth depending on plant water status. Under water stress, ABA depresses both shoot and root growth, but the overall effect is a dramatic increase in the root:shoot ratio, which facilitates water conservation.
TABLE 1. Mutations Affecting ABA Biosynthesis and Signal Transduction Pathway in Plants
Plant species Mutations
| Plant species | Mutations | Gene product/function |
|---|---|---|
| ABA-deficient mutants Zea mays Z. mays Chlamydomnas reinhardtii Arabidopsis thaliana A. thaliana A. thaliana A. thaliana Nicotiana plumbaginifolia N. plumbaginifolia N. plumbaginifolia N. plumbaginifolia Lycopersicum esculentum L. esculentum L. esculentum Solanum phureja Hordeum vulgare Pisum sativum |
vp2,5,7– 9 vp14 M526 aba1 aba2 aba3 aao3 aba2 aba1/ckr1 aba2 cnxA notabilis flacca sitiens droopy nar2a wilty |
Carotenoid biosynthesis 9-cis-Epoxycarotenoid dioxygenase ABA xanthophyll biosynthesis Zeaxanthin epoxidase Xanthoxin oxidase Molybdenum cofactor biosynthesis Aldehyde oxidase Zeaxanthin epoxidase Molybdenum cofactor biosynthesis ABA xanthophyll biosynthesis Molybdenum cofactor biosynthesis 9-cis-Epoxycarotenoid dioxygenase Molybdenum cofactor biosynthesis Aldehyde oxidase Aldehyde oxidase Molybdenum cofactor biosynthesis ND |
| ABA-insensitive
mutants A. thaliana A. thaliana A. thaliana A. thaliana A. thaliana A. thaliana A. thaliana A. thaliana Z. mays Z. mays Hordeum vulgare Craterostigma plantagineum |
abi1 (SD) abi2 (SD) abi3 abi4 abi5 axr2 (D) gca1– 8 gpa1 vp1 rea cool (ND) cdt-1 (D) |
Type 2C protein phosphatase Type 2C protein phosphatase Seed-specific putative transcription factor Transcription factor bZIP transcription factor Auxin response factor (allelic to the auxin mutant iaa7) ND Heterotrimeric G-protein a-subunit Seed-specific bZIP transcription factor ND ND Regulatory RNA or short peptide |
| ABA-hypersensitive
mutants A. thaliana A. thaliana A. thaliana A. thaliana A. thaliana A. thaliana A. thaliana A. thaliana A. thaliana A. thaliana |
abh1 bri1 era1 era2 era3 fiery1 jar1 jin4 hyl1 sax |
Subunit of a nuclear RNA cap-binding complex Steroid receptor kinase Farnesyltransferase b-subunit ND Novel transmembrane protein (allelic to the ethylene mutantein2) Inositol polyphosphate 1-phosphate ND ND Double-stranded RNA-binding protein ND |
E. ABA Mediates Wound Responses
After mechanical wounding, a specific set of defense- related proteins, such as protease inhibitors I and II, cathepsin D inhibitor, and threonine deaminase, accumulate both at the site of injury and systemically throughout the plant. ABA, together with jasmonic acid, appears to play a role in the induction of these genes.
THE ABA SIGNAL TRANSDUCTION PATHWAY
Clues as to how the ABA signal is transduced to mediate its physiological and developmental pro- cesses are now beginning to emerge. It should be emphasized that although many individual com- ponents have been identified mainly by molecular genetic approaches, the complete network has not yet been elucidated.
A. Receptor(s)
ABA is thought to initiate its effects by binding to a receptor(s) that triggers the signal transduction cascade.
Currently, the identity of the receptor(s) is unknown. Cells may possess at least two sites of ABA perception, one of which is located at the plasma membrane and is triggered by extracellular ABA. Biophysical studies indicate that ABA effects in stomatal guard cells also involve intracellular recep- tors accessible to the protonated form, ABAH, which readily permeates membranes.
B. Downstream Signaling Events
Recently, considerable insights have been gained into the identities of molecular components of the com- plex signaling network that mediates the actions of ABA. In particular, ion channels and fairly ubiquitous small second messengers have been implicated in ABA action.
1. Ion Channels Regulated by ABA Control Stomatal Aperture
Electrophysiological studies, either by whole cell impalement or by patch clamping of the plasma membrane of guard cell protoplasts or isolated vacuoles, have identified a number of membrane ion channels.
The sequence of events in ABA-induced stomatal closure is thought to be the following:
(1) ABA induces release of Ca 2+ into the cytosol from an internal store, e.g., the vacuole.
(2) The resultant increase in cytosolic Ca 2+ inhibits plasma membrane H + pumps and inward K + (K) in + channels, but activates two types of plasma membrane anion-efflux channels. One of these shows voltage-dependent slow activation (S-type), whereas the other shows rapid transient activation (R-type). The two types may reflect different states of a single channel.
(3) The conjugate actions of these channels lead to a transient or sustained depolarization and the alkalinization of the guard cell cytoplasm, which
(4) deactivates K + in + channels and also contributes to the opening of voltage-gated K + out channels. The ensuing long-term efflux of both anions and K + from guard cells contributes to loss of turgor and to stomatal closure.
2. Ca 21 Channels
Considerable evidence indicates that ABA produces repetitive, transient increases or oscillations in intracellular Ca 2+ levels. These encode information required for stomatal closure. Ca 2+ induced [Ca 2+ ] cyt oscillations include a repetitive Ca 2+ influx across the plasma membrane coupled to Ca 2+ release from an intracellular compartment. Stomatal closure is abol- ished in guard cells when a nonoscillating Ca 2+ plateau is imposed experimentally.
Many other stimuli responsible for stomatal closure (cold shock, oxidative stress, and increases in CO 2 ) also cause [Ca 2+ ] cyt elevations. Anion channel regulation and stomatal movement phenotypes of Arabidopsis abi1-1 or abi2-1 mutants are suppressed by experimentally elevating [Ca 2+ ] cyt .
The mechanisms by which ABA activates guard cell plasma membrane Ca 2þ channels remain unknown. In Arabidopsis guard cells, ABA causes a rapid increase of reactive oxygen species (ROS) that activate hyperpolarization-activated Ca 2+ -permeable channels. ROS-induced stomatal closure and Ca 2+ activation are abolished in the ABA-insensitive mutant gca2.
The origin of the Ca 2+ required to elevate [Ca 2+ ] cyt in response to ABA is unclear, but is probably mediated by inositol 1,4,5-trisphosphate (InsP 3 ) and/or cyclic ADP-ribose (cADPR). RAB18 expression in Arabidopsis suspension culture cells requires rapid ABA-induced Ca 2+ influx and S-type anion channel activation. ABA-induced membrane depolarization in radish seedlings and tobacco epidermal and mesophyll cells indicates that these mechanisms are of general importance for ABA signaling in different cell types. A Ca 2+ -independent pathway also appears to exist.
3. H 1 Channels
Inhibition of the plasma membrane H þ -ATPase mediated by both cytosolic alkalinization and the increase in cytosolic Ca 2+ may also contribute to ABSCISIC ACID 6 membrane depolarization. The origin of ABA- induced cytosolic alkalinization is unknown.
4. Cyclic Nucleotides (cAMP, CGMP, CADPR)
Cyclic ADP-ribose (cADPR) plays a central role in ABA responses. Microinjection of hypocotyl cells of the tomato aurea mutant with both potential intermediates in the ABA signaling cascade and fusions of the Arabidopsis RD29A and KIN2 promoters to a reporter gene suggested that ABA triggers a transient accumulation of cADPR, which induces a release of Ca 2+ from internal stores such as vacuoles and the endoplasmic reticulum. Microinjec- tion of mutant abi1-1 protein inhibited ABA-, cADPR-, and Ca 2+ -induced gene expression, and these effects were reversed by an excess of WT ABI1 protein.
Other cyclic nucleotides may also act in a Ca 2þ - dependent stomatal opening pathway. For example, cAMP or the membrane-permeable cyclic GMP analog 8-Br-cGMP stimulates stomatal opening. cGMP-induced stomatal opening is inhibited by chelation of external Ca 2þ or by inhibitors of intracellular Ca 2þ release.
5. Lipid-Derived Second Messengers
Various lines of evidence suggest that ABA stimulates phosphoinositide metabolism. ABA-treated guardcell protoplasts showed a slight increase in InsP 3 . The release of caged InsP 3 into the cytosol of guard cells caused [Ca 2+ ] cyt increases, inhibition of K þ in channels, and stomatal closure.
The Arabidopsis fry1 (fiery1) mutant, which is defective in an inositol polyphos- phate 1-phosphatase, accumulates more InsP 3 than WT plants after ABA treatment and is hypersensitive to ABA in germination and gene expression assays. Similarly, overexpression of a different InsP 3 phos- phatase blocked the inhibition of germination and seedling growth in Arabidopsis.
Overexpression of a stress- and ABA-inducible phosphatidylinositol- specific phospholipase C (PI-PLC) in Arabidopsis suggests that although increased InsP 3 levels are necessary for maximal ABA-induced gene expression in vegetative tissues, the AtPLC1 isoform is normally latent and probably participates in secondary ABA responses.
A reduction in InsP 3 levels in transgenic lines expressing antisense AtPLC1 correlated with their insensitivity to ABA in germination and seedling growth assays. ABA also stimulates production of myo-inositol- hexakisphosphate (InsP 6 ) in guard cells to a greater extent than InsP 3 . InsP 6 inhibits K + in channels in a Ca 2+ -dependent manner with greater efficiency than InsP 3 . Whether InsP 6 causes [Ca 2+ ] cyt elevations and whether both messengers function in the same or separate signaling branches is unknown.
Phosphatidic acid (PtdOH) generated from phos- pholipase D (PLD) increases transiently following ABA treatment of Vicia faba guard cells. PtdOH promotes stomatal closure and inactivates K þ in chan- nel currents but does not elicit a [Ca 2þ ] cyt increase, suggesting that PLD acts either in a parallel Ca 2þ - independent pathway or downstream of Ca 2þ release.
Both U-73122 (a PI-PLC inhibitor) and 1-butanol (a PLD inhibitor) only partially inhibit ABA-dependent stomatal closure, and simultaneous application of both inhibitors does not have additive effects. Thus, PLC and PLD appear to act in the same pathway that requires the cooperation of an additional pathway(s) to attain the complete effects of ABA.
This may be mediated by cADPR, since simultaneous application of 1-butanol with the cADPR antagonist nicotina- mide increases the extent to which ABA-induced stomatal closure is reduced. Sphingosine-1-phosphate (S1P) is another lipid- derived Ca 2þ -mobilizing agent capable of inducing stomatal closure. An increase in S1P levels occurs in leaves following drought stress, but disruption of S1P production causes only partial inhibition of ABA- induced stomatal closure.
6. Protein Kinases and Protein Phosphatases
Phosphorylation/dephosphorylation events are cen- tral mediators in ABA signaling. In guard cells, the Ca 2+ signal is possibly relayed by specific protein kinases and phosphatases.
Protein kinase inhibitors abolish the activation of S-type anion channels and thus block ABA-induced stomatal closure. Reciprocally, the protein phosphatase inhibitor okadaic acid (OKA) maintains guard cell S-type channels in the active state. In-gel phosphorylation assays demon- strated that ABA rapidly activates a Ca 2+ -indepen- dent 48 kDa Ser/Thr protein kinase in V. faba guard cells. ABA fails to activate anion channels or induce stomatal closure in guard cells that express a dominant loss-of-function allele of this kinase.
Several stress- and ABA-inducible protein kinases have been identified. In epidermal peels of Pisum sativum, the ABA-induced accumulation of a DHN transcript was reduced by K-252a (an inhibitor of Ser/Thr protein kinases) and also by OKA or cyclosporin A (an inhibitor of Ser/Thr protein phosphatases type 2B). In barley aleurone proto- plasts, the stimulation of a MAP kinase activity appeared to be correlated with the induction of RAB16 transcript. OKA inhibited the induction ABSCISIC ACID 7 of HVA1 and RAB16 transcripts by ABA, and phenylarsine oxide (an inhibitor of Tyr protein phosphatases) blocked RAB16 induction.
The analysis of abi1 and abi2 has shed new light on the involvement of phosphorylation events in ABA signaling. These two dominant ABA-insensitive mutants, originally isolated in a screen for mutants able to germinate and grow in nonpermissive ABA concentrations, have phenotypes reminiscent of ABA deficiency viz. reduced seed dormancy, improper regulation of stomatal aperture, and decreased expression of various ABA-inducible genes. ABI1 and ABI2 encode Ser/Thr protein phosphatases type 2C (PP2C).
The dominant mutant alleles abi1-1 and abi2-1 have point mutations that substitute a conserved Gly with Asp, probably disrupting the conformation of a site required for Mg 2þ -binding or phosphatase activity. Several downstream responses to ABA are impaired in abi1-1 and abi2-1, including K þ out and K þ in channel regulation, anion channel activation, and increases in [Ca 2+ ] cyt . Because the mutations are dominant, it remains unclear whether the ABI1 and ABI2 are positive or negative regulators of ABA signaling or, indeed, whether they affect ABA signaling at all in WT plants.
However, because intragenic revertants of abi1-1 and abi2-1 have reduced or no phosphatase activity in vitro and a double mutant of both revertants is hypersensitive to ABA, ABI1 and ABI2 are probably negative regulators of ABA signaling.
Accordingly, overexpression of WTABI1 in maize mesophyll protoplasts blocks ABA regulation of gene expression. The precise roles of kinases and phosphatases in ABA signaling and the identities of their protein substrates have not been clearly established.
7. Farnesylation
Although researchers have focused mainly on posi- tively acting components of the ABA signaling pathway, inactivation of negative regulators of ABA signaling should result in an enhanced response to ABA. An Arabidopsis mutant era1 (enhanced response to ABA) was isolated based on its inability to germinate in the presence of low concentrations of ABA (0.3 mM) that do not inhibit germination of WT seeds.
The era1 mutation markedly increases seed dormancy and ABA hypersensitive activation of S-type anion currents in this mutant increases stomatal closure, reducing water loss during drought. The ERA1 gene encodes the b-subunit of a hetero- dimeric farnesyltransferase. Farnesyltransferases cat- alyze the attachment of a 15-carbon farnesyl lipid to C-terminal target sequences, which localizes specific soluble signaling proteins to membranes. In addition to enhancing ABA signaling, loss of ERA1 function affects several other signaling pathways and develop- mental programs, including meristem development.
Thus, although ERA1 targets are not restricted to ABA action, a factor that normally suppresses ABA responses requires farnesylation. The exact relation- ship between ERA1 and the ABI loci remains unknown.
8. RNA Binding and ABA
Arabidopsis abh1 mutants are hypersensitive to ABA- mediated inhibition of germination as well as induction of stomatal closure and increases in [Ca 2þ ] cyt . ABH1 is expressed in stomata and encodes a nuclear transcript cap-binding protein that appar- ently functions in a heterodimeric complex. ABH1, by analogy to yeast and mammalian RNA cap- binding proteins, is proposed to regulate the strength of ABA signaling by transcript modification of early signaling components.
9. Heterotrimeric G-Protein Action
Heterotrimeric G-proteins are central to many signaling processes. Arabidopsis contains only a single gene (GPA1) encoding a prototypic Ga subunit. ABA-mediated inhibition of stomatal open- ing, but not ABA-controlled promotion of stomatal closure, is impaired in gpa1 null mutants. GPA1 is required for negative regulation of K þ in channels and the pH-independent activation of anion channels.
10. Role of the Actin Cytoskeleton in Stomatal Movements
A reorganization of the actin cytoskeleton of guard cells has been observed after ABA treatment. Cyto- chalasin D (an actin filament-depolymerizing agent) activates K þ in channels, while phalloidin (an actin filament stabilizer) inhibits K þ channel currents. ABA treatments reorganize actin cytoskeleton architecture from a radial arrangement to a randomly oriented and fragmented pattern.
A small Arabidopsis GTP- binding protein, AtRac1, is a negative regulator in ABA-induced actin reorganization. The inactivation of AtRac1 by ABA is impaired in abi1-1.
C. Regulation of Gene Expression by ABA
ABA regulates the expression of numerous genes during embryogenesis and seed maturation as well as under stress conditions such as heat shock, low temperature, drought, and high salinity.
1. ABA-Inducible Genes
The use of ABA-deficient and ABA-insensitive mutants has demonstrated that ABA contributes to the regulation of numerous genes involved in seed maturationand/ortheresponseofvegetativetissuesto hyperosmotic stress.
Characterization of the promo- ters of ABA-responsive genes has enabled identifi- cation of the cis- and trans-acting elements that act at the termini of branches in the ABA signaling cascade. Considerable evidence indicates the existence of ABA-independent dehydration and cold-induced sig- naling pathways.
2. Cis-Acting Elements
Gene activation is mediated by the binding of transcription factors to ABA-responsive elements (ABREs) located in the promoters of ABA-induced genes. To date, more than 20 functional ABREs have been found in ABA-inducible genes that are abun- dantly expressed in desiccating seeds and/or are responsive to drought stress and ABA in vegetative tissues.
The first type of ABRE defined was a sequence of 8–10 bp that shares a conserved ACGT core motif, named the G-box. The sequence flanking the ACGT core is important for in vivo and in vitro function. Some ACGT elements confer developmental and tissue-specific expression on a minimal promoter. In a natural promoter context, an ABRE functions with a coupling element (CE).
ABA-responsive complexes comprising an ABRE and a CE can confer ABA- inducible transcription upon a minimal promoter. The sph element, first identified in the promoter of the C1 gene involved in anthocyanin synthesis in maize endosperm, is a second category of cis-acting element distinct from the G-box.
3. Trans-Acting Factors
Yeast one-hybrid assays to identify ABRE-binding proteins (AREBs) have enabled cloning of several homologous transcription factors of the basic leucine zipper (bZIP) family. ABA-regulated transcription factors of the homeodomain leucine zipper, basic helix-loop-helix leucine zipper, and MYB classes have also been identified. AREBS are capable of activating reporter genes fused to ABREs and their induction by ABA at the transcript level frequently precedes the inductionofotherABA-responsivegenes.
Arabidopsis ABI5, the only bZIP AREB recovered in a genetic screen, is also subject to post transcriptional modification by ABA. Maize VP1 and Arabidopsis ABI3 appear to be orthologous seed-specific transcriptional activators, the loss of which affects several aspects of seed maturation, including the expression of storage proteins and LEA genes. VP1/ABI3-like proteins appear to activate transcription by distinct mechanisms depending on the target cis elements. VP1 interacts directly with the sph element of the C1 promoter and acts on ABREs via association with a distinct trans-acting factor(s).
D. Novel Genetic Screens
Quantitative and mechanistic characterization of new signaling mutants is necessary for a complete molecular understanding of the ABA signaling cascade. Several mutants have been isolated in screens for deregulated ABA control of ABREs fused to reporter genes.
Eight gca (growth control by ABA) mutants are characterized by reduced sensitivity to the inhibition of seedling growth by exogenous ABA and aberrant stomatal regulation. An elegant screen that uses small differences in leaf temperature to distinguish transpiration rates in mutants and WT plants is likely to identify new elements which mediate ABA action in guard cells. Screens for enhancer or suppressor mutations offer one approach to identify genes which interact genetically with known participants in ABA signaling.
BIOTECHNOLOGICAL FEATURES
Fresh water scarcity is currently one of the principal threats to global food security. Plants account for approximately 65% of global fresh water use. Losses in agricultural yields resulting from the desiccation of crops and horticultural plants during periods of drought have severe social and economic repercussions. Unfortunately, because of the high cost of synthesis and its instability in UV light, there are no practical uses of ABA. However, synthetic ABA analogs such as the acetyleneacetal-type compounds LAB 173 711 and LAB 144143 reduce crop water use and increase cold-hardiness.
Engineering the ABA signal transduction network in guard cells to control CO 2 intake and water loss could contribute substantially to more sustainable water use under adverse environmental conditions. The manipulation of seed maturation and dormancy in certain species by modification of ABA-regulated developmental pro- grams may also be of considerable agricultural significance.
CONCLUSIONS
Recent advances have filled in many gaps concerning the biochemistry and subcellular localization of ABA synthesis as well as demonstrating the unquestionable involvement of ion channels, cytosolic pH, protein (de)phosphorylation, and cADPR- and phosphoinosi- tide-mediated increases in [Ca 2þ ] cyt in transducing the ABA signal.
Substantial progress has been made in characterization of the terminal signaling elements involved in ABA-mediated transcriptional regulation. Nonetheless, the mechanism(s) of ABA perception and early signaling events that result in cADPR synthesis or InsP 3 release remain to be resolved.
Considering the multitude of physiological responses modulated by ABA, it will be interesting to assess the extent of overlap in the signaling events involved in well-characterized ABA effects such as the regulation of stomatal closure, the inhibition of seed germina- tion, and the induction of stress-responsive gene expression.
The striking degree of phenotypic pleiotropy observed in many mutants recovered in screens for altered sensitivity to ABA indicates extensive overlap between ABA action and other signaling pathways. Better insight into the regulation of ABA concentrations and the cellular capacity for response to ABA will provide a more complete picture of its importance to growth and development throughout the plant life cycle.